Functional Groups: Ethers and Esters
Ethers
Ethers are primarily a carbon parent chain separated by an oxygen
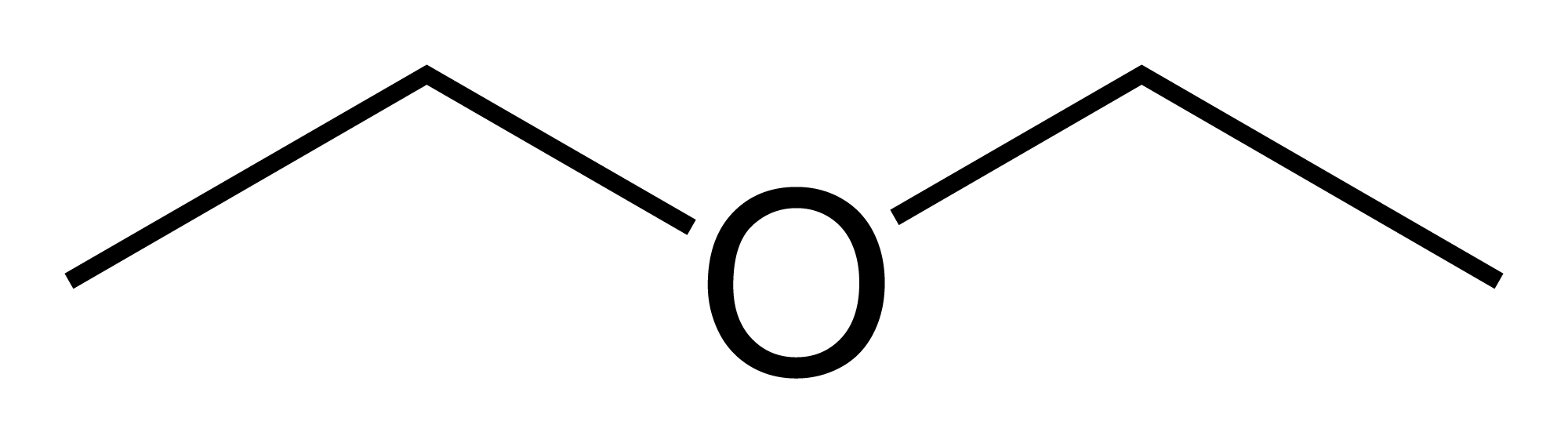 diethyl ether
diethyl ether dimethyl ether
dimethyl etherEsters
Esters are primarily the combination of an ether and a carboxylic acid. Together, the create a ester and water. The table shows how to create an ester (esterfication)

You would name an ester the same way you would name an ether, but to name the parent chain, you need to identify which side has the double bonded oxygen, use that side as your parent chain, and use the ending -oate.


0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home